Service hotline:+86-(0)-18115476705
FRET Labeling
Fluorescence resonance energy transfer (FRET) is a rapid, highly sensitive, and straightforward method that allows the distance-dependent interaction between the excited states of two distinct dye-linked molecules to be detected. The excitation is transferred from a donor to an acceptor without emitting a photon. The majority of applications require two although it is possible to perform FRET using a single type of dye. A practical measure of FRET efficiency is the distance (Förster-radius) at which the rate of energy transfer equals the rate of donor fluorescence.FRET is used to measure the transfer of energy from the donor that is excited initially (dye 1) to an acceptor (dye 2). The emit wavelength of a donor generally overlaps with the absorption wavelength of the acceptor. The energy transfer occurs when the donor and acceptor dyes are in close proximity (10–100Å), depending on the chemical structure of the acceptor. There are two ways: • a) If the acceptor is a dark quencher, the energy transferred energy could be converted into molecular vibrations • b) If the acceptor is fluorescent, the transferred energy might be emitted as light with a longer wavelength .
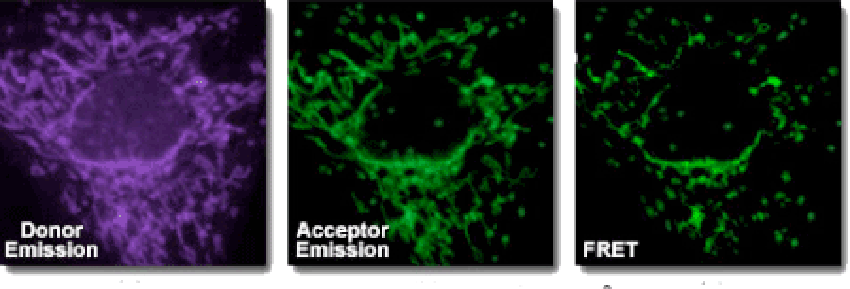
We can use FRET to study peptidase specificity because FRET allow reactions to be monitored continuously, allowing the enzymatic activity to be determined rapidly. The peptide bonds between the donor/acceptor pair can be cleaved, which generates a fluorescent signal to allow nanomolar concentrations of enzyme activity to be measured. When intact, FRET peptides quench internal fluorescence; however, the cleavage of a peptide bond between the donor/acceptor pair releases a fluorescent signal that can be detected continuously, allowing enzyme activity to be quantified. FRET peptides are used as suitable substrates in many different enzyme studies such as kinetic and functional characterization of peptidases, proteases, kinases, and phosphatases, Screening and detection of novel proteolytic enzymes, Conformational investigation of peptide folding.
Dabcyl
Edans
336
490
Dansyl
Trp
336
350
DNP
Trp
328
350
DNP
MCA
328
393
DNP
Abz
328
420
Tyr (NO2)
Abz
320
420
Tryptophan
Dansyl
2.1
IAEDANS (1)
DDPM (2)
2.5-2.9
BFP
DsRFP
3.1-3.3
Dansyl
FITC
3.3-4.1
Dansyl
Octadecylrhodamine
4.3
CFP
GFP
4.7-4.9
CF (3)
Texas Red
5.1
Fluorescein
Tetramethylrhodamine
4.9-5.5
Cy3
Cy5
5.0
GFP
YFP
5.5-5.7
BODIPY FL (4)
BODIPY FL (4)
5.7
Rhodamine 6G
Malachite Green
6.1
FITC
Eosin Thiosemicarbazide
6.1-6.4
B-Phycoerythrin
Cy5
7.2
Cy5
Cy5.5
>8.0
(1): 5-(2-iodoacetylaminoethyl)aminonaphthalene-1-sulfonic acid
(2): N-(4-dimethylamino-3,5-dinitrophenyl) maleimide
(3): carboxyfluorescein succinimidyl ester
(4): 4,4-difluoro-4-bora-3a, 4a-diaza-s-indacene
Copyright © Nanjing TGpeptide Biotechnology Co.,Ltd.